- Home
- Research
Research Projects
Regulation of muscle plasticity by mechanical force

It is becoming clear that various physical stimuli (mechanical stress) are loaded on living bodies due to gravity, physical exercise, pulsation and blood flow, and optimization of biological functions and long-term reconstruction of biological tissues (remodeling) is being carried out in response to those stresses.
For example, compressive stress loaded on bones in the longitudinal direction by gravity generate hydrostatic pressure in artificial cartilage and it controls extracellular matrix production such as collagen during walking and running. On the contrary, in a microgravity environment where mechanical stress is not loaded or drastically reduced environments including immobility, inactivity, disuse muscle atrophy and bone loss (osteoporosis) are induced. These phenomena are very dynamic and are also very interesting from biological point of view. At the same time, these phenomena are also thightly-related to today's problems in our aged society.
In our laboratory, we are takeing advantage of cultured cells and experimental animals to explore how mechanical stress controls skeletal muscle plasticity.
Related achivements
Akimoto et al. Skeletal muscle adaptation in response to mechanical stress in p130Cas-/- mice. Am J Physiol Cell Physiol, 304(6), C541-C547, 2013Horiuchi et al. Mechanical stretch maintains Nanog expression through PI3K/Akt signals in mouse embryonic stem cells. Exp Cell Res, 318(14), 1726-1732, 2012
Akimoto et al. Mechanical stretch inhibits myoblast-to-adipocyte differentiation through Wnt signaling. Biochem Biophys Res Commun, 329(1), 381-385, 2005
Regulation of muscle plasticity by non-cording RNAs

In our laboratory, we are approaching the control of skeletal muscle plasticity by miRNA mainly using cultured cells and experimental animals.
Related achivements
Oikawa et al. An inducible knockout of Dicer in adult mice does not affect endurance exercise-induced muscle adaptation. Am J Physiol Cell Physiol, 316(2), C285-C292, 2019Wada et al. MicroRNA-23a has minimal effect on endurance exercise-induced adaptation of mouse skeletal muscle. Pflügers Archiv, 467(2), 389-398, 2015
Russell et al. Regulation of miRNAs in human skeletal muscle following acute endurance exercise and short term endurance training. J Physiol, 591(Pt 18), 4637-4653, 2013
Russell et al. Disruption of skeletal muscle mitochondrial network genes and miRNAs in amyotrophic lateral sclerosis. Neurobiol Dis, 49C, 107-117, 2012
Wada et al. Translational suppression of atrophic regulators by miR-23a integrates resistance to skeletal muscle atrophy. J Biol Chem, 286(44), 38456-38465, 2011
Molecular mechanism of exercise-induced health benefits
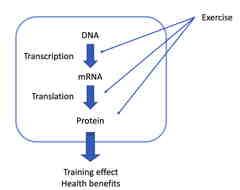
In our laboratory, we are approaching with experimental animals to clarify the molecular mechanism underlying the health benefits of exercise.
Related achivements
奥津光晴, 秋本崇之. 運動がPgc-1αを介してヘルス・ベネフィットをもたらす分子メカニズムを探る. 臨床スポーツ医学, 30(10), 1283-1287, 2013Aizawa et al. Endurance exercise training enhances local sex steroidogenesis in skeletal muscle. Med Sci Sports Exerc, 43(11), 2072-2080, 2011
Aizawa et al. Acute exercise activates local bioactive androgen metabolism in skeletal muscle. Steroid, 75(3): 219-23, 2010
Akimoto et al. Functional interaction of regulatory factors with the Pgc-1α promoter in response to exercise by in vivo imaging. Am J Physiol Cell Physiol, 295(1), 288-292, 2008
Skeletal muscle tissue engineering
On the other hand, it has been clarified that there is an extreme difference in function and morphology between myotubes and adult skeletal muscles. In our laboratory, we are constructing skeletal muscle tissue in vitro by applying tissue engineering technology and seeking approaches to use it for muscle biology research.